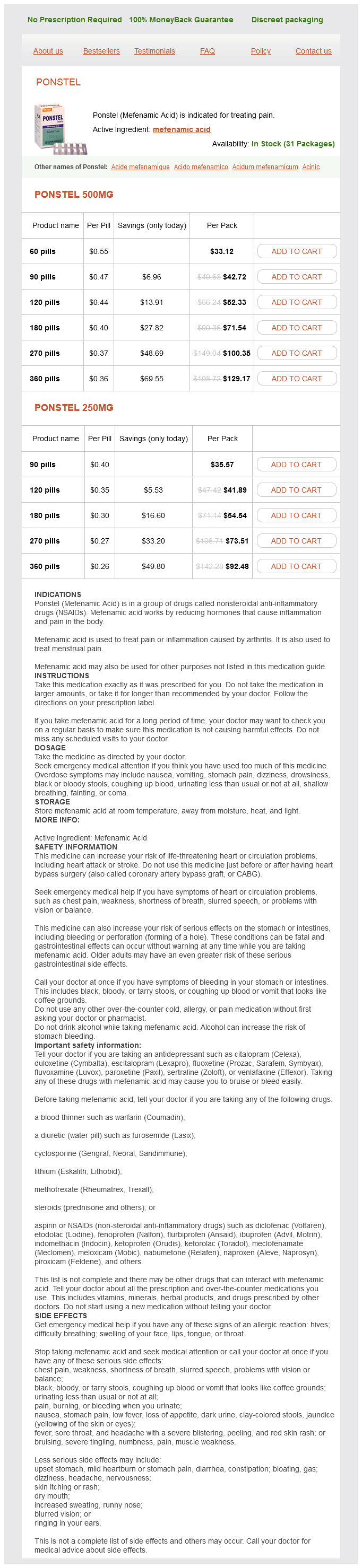
Ponstel Dosage and Price
Ponstel 500mg
- 60 pills - $33.12
- 90 pills - $42.72
- 120 pills - $52.33
- 180 pills - $71.54
- 270 pills - $100.35
- 360 pills - $129.17
Ponstel 250mg
- 90 pills - $35.57
- 120 pills - $41.89
- 180 pills - $54.54
- 270 pills - $73.51
- 360 pills - $92.48
Olaratumab therapy should be held in the setting of neutropenic fever/infection or when grade 4 neutropenia lasts longer than 1 week muscle relaxant little yellow house 250 mg ponstel order with visa. No dose reduction is necessary in patients with mild or moderate renal dysfunction. The drug has not been studied in patients with severe renal dysfunction or in those with end-stage renal disease. Infusion-related symptoms, with fever, chills, urticaria, flushing, fatigue, headache, bronchospasm, dyspnea, angioedema, and hypotension. Approximately 44% of an administered dose eliminated in feces and 37% eliminated in urine. Patients should be weighed and monitored regularly for symptoms and signs of fluid retention, especially when using higher drug doses and in patients age > 65 years. Monitor blood glucose levels frequently in patients with diabetes or in patients with risk factors for diabetes. Should be avoided in patients with poorly controlled diabetes until good glycemic control has been established. Gastrointestinal toxicity with diarrhea, nausea/vomiting, abdominal pain, and anorexia. Chemotherapeutic and Biologic Drugs 387 O Oral bioavailability is on the order of 92%. The mean exposure of each of these metabolites was approximately 10% of the exposure of parent drug at steady state. Renal elimination of parent drug and its metabolites account for only about 14% of an administered dose. Dose of warfarin may require careful adjustment in the presence of osimertinib therapy. Has not been studied in patients with moderate or severe hepatic dysfunction, and caution should be used in this setting. Has not been studied in patients with severe renal dysfunction, and caution should be used in this setting. Widely distributed to all tissues, with a 50-fold higher volume of distribution than cisplatin. As observed with cisplatin, oxaliplatin undergoes aquation reaction in the presence of low concentrations of chloride. Use with caution in patients with abnormal renal function, especially when CrCl < 20 mL/min, as the drug has not been studied in this setting of severe renal impairment. Careful neurologic evaluation should be performed before starting therapy and at the beginning of each cycle, as the dose-limiting toxicity of oxaliplatin is neurotoxicity. Calcium/magnesium infusions (1 g calcium gluconate/1 g magnesium sulfate) prior to and at the completion of the oxaliplatin infusion can be used to reduce the incidence of acute neurotoxicity. There is no evidence that these infusions impair the clinical activity of oxaliplatin. May lengthen oxaliplatin infusion from 2 to up to 4 hours to reduce the development of acute neurotoxicity. Chemotherapeutic and Biologic Drugs 391 O Neurotoxicity with acute and chronic forms. Acute toxicity is seen in up to 80%85% of patients and is characterized by a peripheral sensory neuropathy with distal paresthesia and visual and voice changes, often triggered or exacerbated by cold. Dysesthesias in the upper extremities and laryngopharyngeal region with episodes of difficulty breathing or swallowing can be observed and usually within hours or 13 days after therapy. Chronic toxicity is dose-dependent, with a 15% and > 50% risk of impairment in proprioception and neurosensory function at cumulative doses of 850 and 1,200 mg/m2, respectively. Oxaliplatin-induced neuropathy appears to be more readily reversible than cisplatin and returns to normal within 34 months of discontinuation of oxaliplatin. Results in enhanced drug efflux, with decreased intracellular accumulation of drug. Distributes widely to all body tissues, including third-space fluid collections such as ascites. Renal clearance is relatively minor, with less than 10% of drug cleared via the kidneys. Terminal elimination half-life ranges from 9 to 50 hours depending on the schedule of administration. Cyclophosphamide-Myelosuppression is greater when cyclophosphamide is administered before paclitaxel. Patients with abnormal liver function are at significantly higher risk for toxicity. Patients who have received > 6 courses of weekly paclitaxel should be advised to avoid sun exposure of their skin as well as their fingernails and toenails, as they are at increased risk for developing onycholysis. Myelosuppression is dose-limiting, and neutropenia is most common with nadir at day 810 and recovery by day 1521. Decreased incidence of neutropenia with 3-hour schedule when compared to 24-hour schedule. Characterized by generalized skin rash, flushing, erythema, hypotension, dyspnea, and/or bronchospasm. Neurotoxicity, mainly in the form of sensory neuropathy with numbness and paresthesias. Transient asymptomatic sinus bradycardia is most commonly observed cardiotoxicity. Use with caution in patients with moderate or severe hepatic impairment, although no specific dose recommendations have been provided.
Duration of Therapy · Drugs that modify platelet activity zyprexa spasms order 250 mg ponstel with visa, lipoprotein concentrations, and neurohormonal systems reduce the risk for coronary events and death. However, treatment with at least one agent that improves the balance between myocardial oxygen demand and supply is usually warranted. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Sangareddi V, Chockalingam A, Gnanavelu G, Subramaniam T, Jagannathan V, Elangovan S. Canadian Cardiovascular Society classification of effort angina: An angiographic correlation. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Meta-analysis of natural therapies for hyperlipidemia: Plant sterols and stanols versus policosanol. Long-term impact of drug-eluting stents versus bare-metal stents on all-cause mortality. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). A report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Effects of early and late administration of angiotensin-converting enzyme inhibitors on mortality after myocardial infarction. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. Efficacy of monotherapy compared with combined antianginal drugs in the treatment of chronic stable angina pectoris: A meta-analysis. Health outcomes associated with antihypertensive therapies used as first-line agents. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. Ultimately, a thrombus composed of fibrin and platelets may develop, resulting in incomplete or complete occlusion of a coronary artery. These practice guidelines are based on a review of available clinical evidence, have graded recommendations based on evidence and expert opinion, and are updated periodically. The clinical significance of serum markers is discussed in greater detail in later sections of this chapter. The latter explains why these plaques only show minimal luminal obstruction, despite being larger than plaques that characterize stable angina which are associated with more severe luminal narrowing. Fibrin stabilizes the clot and traps red blood cells, which gives the clot a red appearance. Associated symptoms include nausea, vomiting, diaphoresis, or shortness of breath. It is characterized by left ventricular dilation and reduced pumping function of the left ventricle, leading to cardiac failure. Accompanying symptoms may include radiation of pain to arm, back, or jaw, nausea, vomiting, diaphoresis, anxiety, or shortness of breath. Laboratory Tests · Troponin I or T are measured at presentation and repeated 23 times at 6- to 8-hour intervals to ascertain heart muscle damage; confirmatory for the diagnosis of an infarction. Indications and contraindications for fibrinolytic therapy are described in the treatment section of this chapter. Patients with a high likelihood of coronary ischemia have a greater risk of adverse cardiac events. The timing of the diagnostic angiography in moderate- to high-risk patients is generally guided by the short-term risk of the patients, with an earlier angiography generally preferred in high-risk individuals. General Approach to Treatment Selecting evidence-based therapies described in the guidelines for patients without contraindications results in lower mortality. Thus, education to patients and their families is paramount to reduce delays in reperfusion. The use of fibrinolytics between 12 and 24 hours after symptom onset should be limited to patients with ongoing ischemia. What adjunctive pharmacotherapy should be administered in the emergency department prior to proceeding to the cardiac catheterization laboratory Generally, a more fibrin-specific agent such as alteplase, reteplase, or tenecteplase is preferred over a nonfibrin-specific agent such as streptokinase. In a large clinical trial, administration of alteplase resulted in a 1% absolute reduction in mortality and cost about $30,000 per year of life saved compared with streptokinase. Contraindicationsb Hypersensitivity, active bleeding, severe bleeding risk Hypersensitivity, active bleeding, severe bleeding risk Dose and Duration of Therapy 160325 mg orally once on hospital day 1. In patients receiving a fibrinolytic or who do not receive reperfusion therapy, administer for at least 14 days (class I recommendation) and up to 1 year.
Should be discontinued in patients with impaired wound healing and withheld prior to surgery muscle relaxant anxiety order line ponstel. If a patient develops wound-healing complications during therapy, discontinue until the wound is fully healed. Should be withheld for urine protein levels that are >2 g over 24 hours and reinitiated at a reduced dose once the urine protein level returns to <2 g over 24 hours. Permanently discontinue if urine protein levels >3 g over 24 hours or in the setting of nephrotic syndrome. No dose adjustments are required in patients with mild or moderate hepatic impairment. The mean relative bioavailability of tablets compared to oral solution is 69% to 83%. Food with a high fat content increases oral bioavailability of parent drug by 48%. The terminal half-life of regorafenib is 28 hours, while the terminal half-lives of the M-2 and M-5 metabolites are approximately 25 and 51 hours, respectively. Regorafenib should be taken at the same time each day with a low-fat breakfast that contains less than 30% fat. No initial dosage adjustment is needed in patients with pre-existing mild or moderate hepatic disease; however, regorafenib has not been studied in patients with severe hepatic disease, and use in this population is not recommended. Sun exposure should be avoided, and periodic dermatologic evaluation is recommended. Regorafenib should be interrupted in patients undergoing major surgical procedures. Skin toxicity in the form of hand-foot syndrome and skin rash occur in up to 45% and 26%, respectively. Decreased retinoblastoma protein phosphorylation (pRb) resulting in reduced E2F expression and signaling. Use with caution in patients with moderate or severe hepatic impairment, as ribociclib has not been studied in these settings. Use with caution in patients with severe renal impairment, as ribociclib has not been studied in this setting. Elimination pathway has not been well characterized, although antibodycoated cells are reported to undergo elimination via Fc-receptor binding and phagocytosis by the reticuloendothelial system. If the first treatment is well tolerated, the starting infusion rate for the second and subsequent infusions can be administered at 100 mg/ hour with 100-mg/hour increments at 30-minute intervals up to 400 mg/ hour. Use with caution in patients with pre-existing heart disease, including arrhythmias and angina, as there is an increased risk of cardiotoxicity. The development of cardiac arrhythmias requires cardiac monitoring with subsequent infusion of drug. Usually occur within 30 minutes to 2 hours after the start of the first infusion, and they usually resolve upon slowing or interrupting the infusion and with supportive care. Risk is increased in patients with high numbers of circulating malignant cells (>25,000/mm3) and/or high tumor burden with bulky lymph nodes. Hyaluronidase has a local and reversible effect by increasing the permeability of the subcutaneous tissue by depolymerizing hyaluronan. Monitor for tumor lysis syndrome, especially in patients with high numbers of circulating cells (>25,000/mm3) or high tumor burden. In this case, the first dose of rituximab can be split into two doses, with 50% of the total dose to be given on days 1 and 2. Usually resolve upon slowing or interrupting the infusion and with supportive care. Characterized by hyperkalemia, hyperuricemia, hyperphosphatemia, hypocalcemia, and renal insufficiency. Induction of cell-cycle arrest in G1- and G2/M-phases and/or apoptosis may then occur. The precise mechanism(s) by which romidepsin exerts its antitumor activity has not been fully characterized. The dose of warfarin may require careful adjustment in the presence of romidepsin therapy. Rucaparib has not been studied in patients with moderate or severe hepatic impairment, and there are no formal recommendations for dosing in these settings. Rucaparib has not been studied in patients with severe renal impairment or in those on dialysis, and there are no formal recommendations for dosing in these settings. Females of reproductive potential should be warned of the potential risk to a fetus and to use effective contraception. Monitor for infusion-related events, which typically occur within 1 day of infusion and are more severe following the second infusion. The concomitant use of chemotherapy and immunosuppressive medications should be avoided, as they have the potential to reduce the efficacy and/or alter the toxicity of sipuleucel-T. Binds to and inhibits smoothened, a transmembrane protein that is involved in Hedgehog signaling. The main route of elimination of parent drug and its metabolites is hepatic, with excretion in feces (70%), with renal elimination accounting for 30% of an administered dose. Dose reduction is not required in patients with mild, moderate, or severe hepatic impairment. Dose reduction is not required in patients with mild or moderate renal impairment. The pregnancy status of female patients must be verified prior to the start of vismodegib therapy given the risk of embryo-fetal death and/or severe birth defects.